Click on the image to view full size
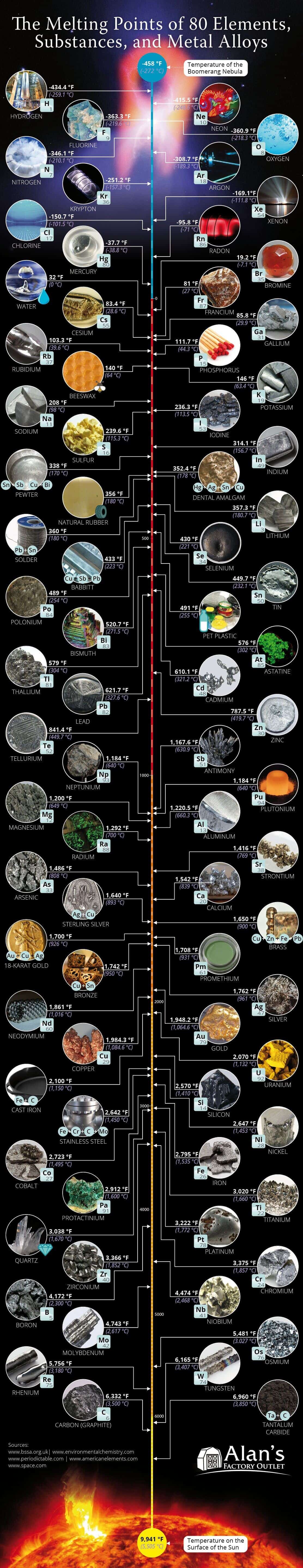
The melting point of a substance is the temperature at which it changes from a solid to a liquid state. The reverse process of a liquid or gas state into a solid is the freezing point, which is the same temperature as the melting point. Keep in mind that pressure is also a key factor in state changes. For example, helium would require a pressure of over twenty times normal atmospheric pressure to freeze.
The coldest known object in the universe is the Boomerang Nebula, which measures in at the temperature of -458 °F (-272 °C).
The element hydrogen’s melting (or freezing) point comes close at -434.4 °F (-259.1 °C). Since hydrogen exists naturally as a gas, it requires immensely cold temperatures to condense it into a liquid. Liquid hydrogen is mainly used for rocket fuel for spacecraft. It has the lowest molecular weight of any known substance and burns with extreme intensity at 5,500°F. It requires extreme, technical care to store and handle hydrogen.
The temperature on the surface of the sun is 9,941 °F (5,505 °C).
The melting point of tantalum carbide comes the closest at 6,960 °F (3,850 °C). It is used in tool bits for cutting and is added to tungsten carbide alloys.
The chemical element with the highest melting point is tungsten at 6,177 °F (3,414 °C).
This property makes tungsten excellent to use as filament in light bulbs. Alloys made with tungsten are often used to make wear-resistant abrasives, cutting tools like knives and drills, and mining, milling, and turning instruments to be used in the metalworking, woodworking, mining, and petroleum industries.
This educational resource was created by Alan’s Factory Outlet, your source for custom-built metal garages for sale and metal carports for sale.


